The Company, faced with a request for an international partnership, expressed the need to assess the feasibility of introducing a new synthesis process in its production plant.
The peculiarity of the activity is due, on the one hand, to the hazardous characteristics of one of the products used in the synthesis, a toxic, highly reactive and unstable gas, and, on the other, to the constraints imposed by the urban context in which the plant is located.
The implementation of the activities required specific in-depth studies:
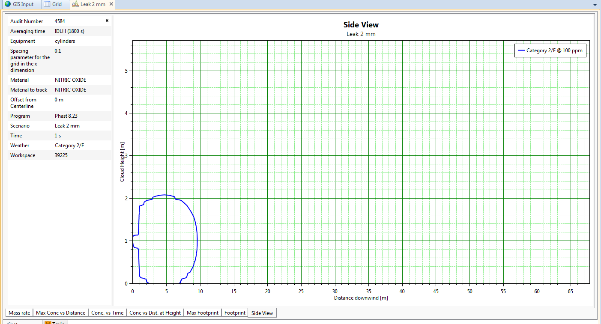
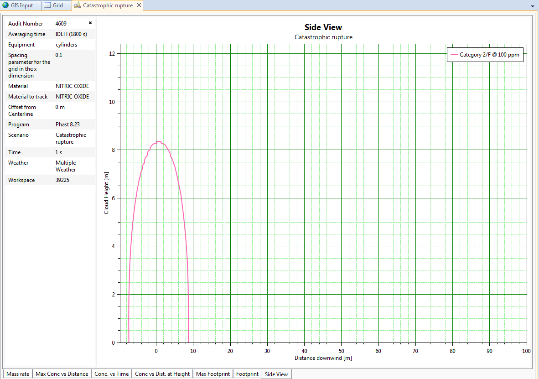
- in estimating consequences associated with toxic gas releases, due to random failures of primary containment systems
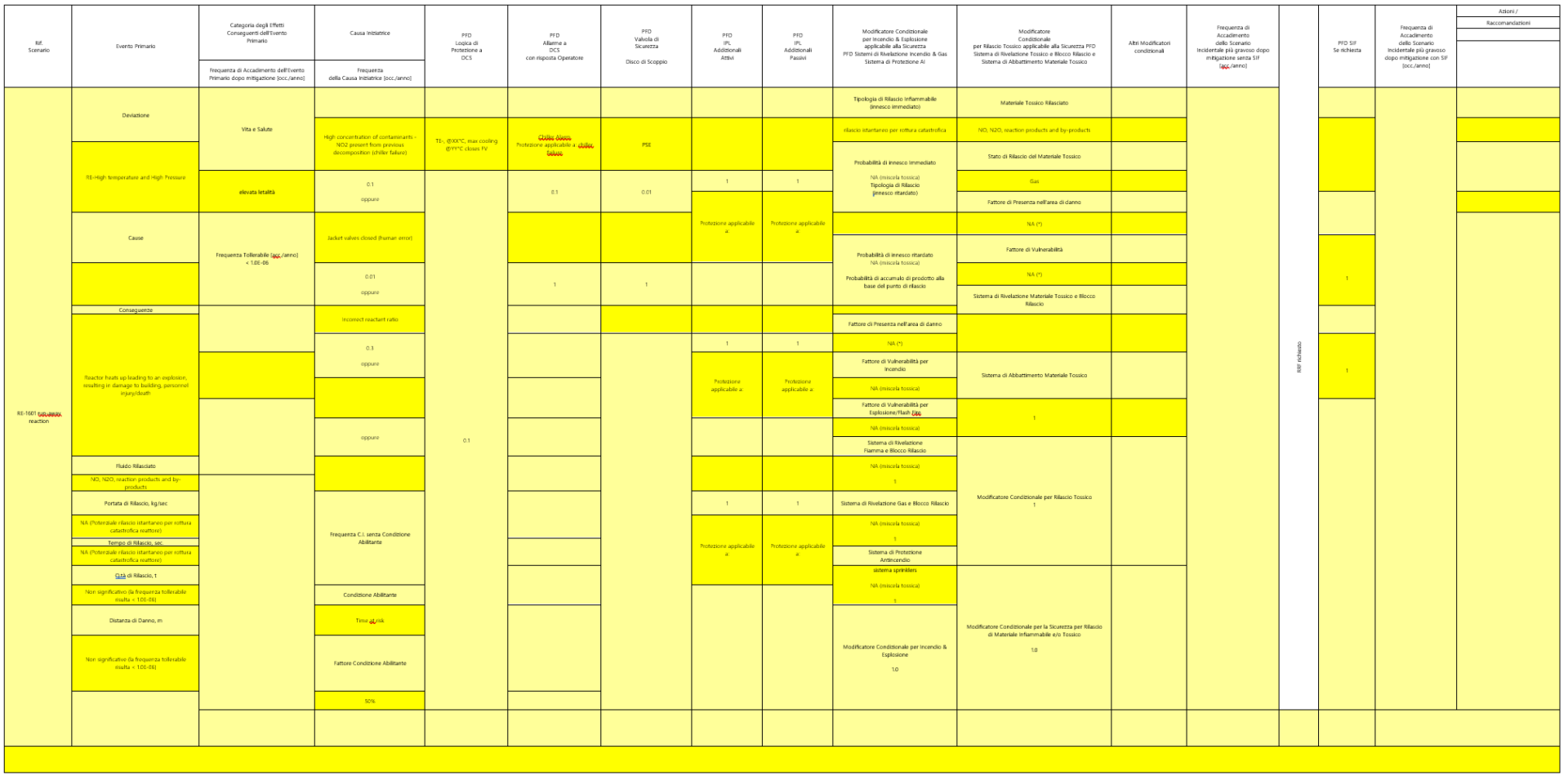
- in estimating the frequency of occurrence of the reactor overpressure hypothesis due to run-away reaction, using a simplified LOPA approach.
The PHAST® simulator from DNV-GL was used for the development of the consequences of gas releases.
The activity also required the assessment of the impact on the profile of the Plant’s subjectivity to the regulations on the control of major accident hazards, Legislative Decree 105/2015 (Seveso “Directive” in Italy), resulting from the introduction of the new synthesis process, in order to propose a series of technical and/or procedural interventions that would allow the Plant’s subjectivity to remain unchanged.